Millions of people in the United States have received COVID-19 vaccines, to date. Currently three vaccines are authorized and recommended for use by the Food and Drug Administration (FDA) to prevent the virus. Two of the vaccines – one produced by Pfizer and the other by Moderna – require 2 shots, 3-4 weeks apart. The Johnson & Johnson (Janssen) vaccine requires just one dose.
The Law Offices of Leah V. Durant & Associates represents clients who suffer injuries as a result of receiving many routine vaccinations. If you suspect that you have been injured by the COVID-19 vaccine, you may be eligible to receive compensation through the federal Countermeasures Injury Compensation Program (CICP). Individuals who are harmed by the COVID-19 vaccine have up to one year from the date of vaccination to file a claim.
Injuries that result from most other vaccines are handled by the National Vaccine Injury Compensation Program (VICP). Historically, the VICP has provided a much easier means for claimants to obtain compensation for their injuries.
Is the COVID-19 vaccine effective?
According to the Centers for Disease Control and Prevention COVID-19 vaccines are effective at preventing individuals from becoming sick with COVID-19. Experts also believe the vaccines may prevent individuals infected with COVID-19 from becoming seriously ill. It may take the body a few weeks following vaccination to build immunity against the virus. Thus, it is possible for individuals to become infected with COVID-19 after having received the vaccination.
Although the COVID-19 vaccine is effective in preventing illness, scientists are still studying how effective the vaccine is at preventing the spread of the virus to others. Preliminary research shows that even after having been vaccinated, individuals may still be capable of carrying the virus. Until it becomes clear whether vaccinated carriers can transmit the disease, individuals should continue to take protective measures such as; mask-wearing, frequent hand-washing, and social-distancing, in order to protect themselves and prevent transmitting the virus to others.
Are the COVID-19 vaccines safe?
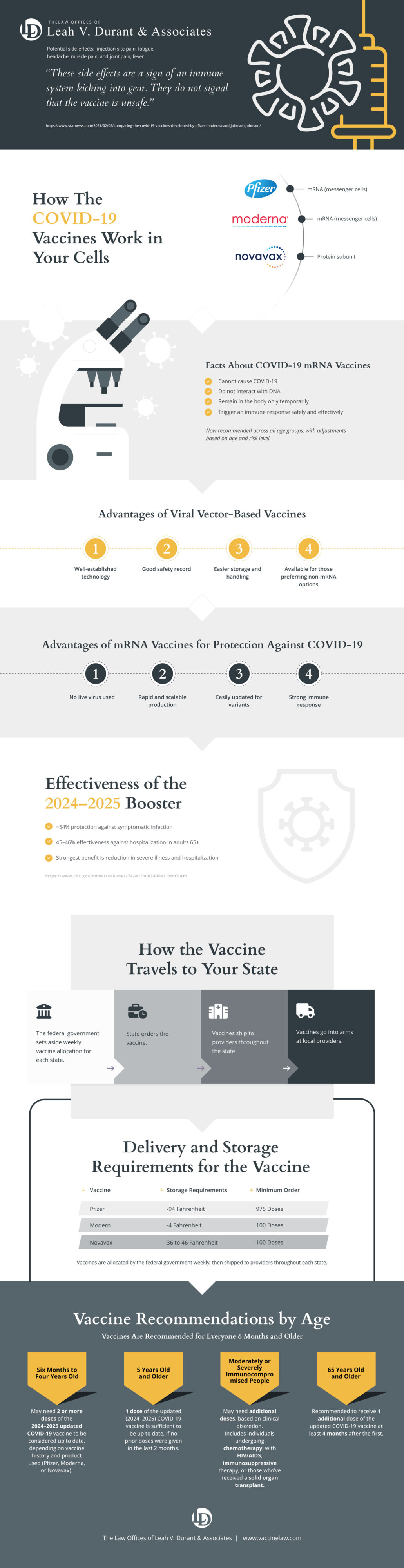
Medical scientists agree that COVID-19 vaccines are safe and effective tools in the fight against the virus. While the vaccines are proven safe, individuals who receive the vaccines may still experience side effects, including chills, tiredness, headache, fever, or pain or swelling at the injection site. These symptoms are normal signs that the body is building protection against the virus, and should resolve without the need for further treatment.
If you are concerned about a reaction you are having to the vaccine, it is advisable that you seek treatment from a medical provider as soon as possible. In addition to seeking medical treatment, individuals may opt to use the after-vaccination health checker, established by the Centers for Disease Control (CDC), called v-safe. V-safe is a smartphone-based application that enables users to obtain personalized health check-ins and information about vaccine reactions, following their receipt of a COVID-19 vaccine.
How does the COVID-19 vaccine work?
Both the Pfizer and Moderna COVID-19 vaccines use what is known as messenger RNA (mRNA) technology. mRNA vaccines deliver genetic information to parts of the body to either erase or overwrite genetic information that is already there. In this case, when you are vaccinated, mRNA vaccines instruct your cells to make certain proteins and begin displaying them on cell surfaces. The theory is that the immune system will recognize the protein does not belong and will then begin building an immune response and making antibodies.
The Johnson & Johnson vaccine works differently. It is a viral vectored vaccine that uses a modified version of a different virus to deliver important instructions to the cells. The modified version is not the COVID-19 virus and is called a “spike protein.” The spike protein triggers the immune system similarly to the other two coronavirus vaccines.
Concerns for the Johnson and Johnson COVID-19 Vaccine
In early April 2021, administration of the Johnson and Johnson vaccine was “paused.” Though not officially recalled or suspended, the US Centers for Disease Control and Prevention and the US Food and Drug Administration suggested suspended use of the Johnson and Johnson vaccine due to reports of blood clots in 6 women. The affected women were between 18 and 48 years of age and suffered the side effect within 6-13 days following administration of the COVID-19 vaccine.
Scientists believe the blood clots are a rare side effect of the drug and are not calling for the vaccine to be recalled. Precautions are merely being taken in order to minimize the risks.
That said, anyone experiencing severe side effects after the Johnson and Johnson COVID-19 shot are strongly encouraged to visit their healthcare provider. Possible side effects include:
- Severe headache
- Shortness of breath
- Abdominal pain
- Leg pain
- Shortness of breath
Should I get the COVID-19 vaccine if I have already had the virus?
According to two new studies, people who have had COVID-19 should get a single dose of the vaccine. The single dose is enough to “turbocharge” an individual’s antibodies and destroy the coronavirus — and even some more infectious variants.
When will I be able to get the vaccine?
The federal government is providing the COVID-19 vaccine free of charge to people living in the United States. Healthcare personnel and residents of long-term care facilities have been the first to receive the vaccine. Currently, frontline essential workers and people over 75 are eligible to receive the vaccine. The next category (phase 1c) will be people aged 65-74, as well as people aged 16-64 with underlying medical conditions, and other essential workers. To see how the vaccine rollout is going in your state, click here.
As vaccine availability increases, CDC vaccination recommendations will expand to include more groups. Each state has its own plan for deciding who will be vaccinated first and how they can receive vaccines. Individuals can contact their local health department to obtain more information concerning COVID-19 vaccination in their area.
Our Vaccine Injury Lawyer Explains the Possibility of an Allergic Reaction
There have been some reports of people experiencing severe allergic reactions (also known as anaphylaxis) after receiving a COVID-19 vaccine. The CDC notes that if you have had a severe allergic reaction to any ingredient in the COVID-19 vaccine, you should not get the Pfizer or Moderna vaccines. Also, if you had a severe allergic reaction after getting the first dose of the COVID-19 vaccine, the CDC recommends that you should not get the second dose. Recommendations are the same if you had an immediate allergic reaction (even if it wasn’t severe), such as hives, swelling, or wheezing.
If you have had an immediate allergic reaction (even if it wasn’t severe) to a vaccine or injectable therapy for another disease, ask your doctor if you should get a COVID-19 vaccine.
Vaccination providers have safeguards in place to prepare for the possibility of a severe allergic reaction to the COVID-19 vaccine. All people who receive the vaccine should be monitored for at least 15 minutes after getting the vaccine. Vaccine sites should have appropriate medications and equipment (such as epinephrine, antihistamines, blood pressure cuffs), ready and available, should reactions occur.
Will I be able to pursue a vaccine injury claim if I suffer an injury following the COVID-19 vaccine?
It is important to understand that if you suffer a severe allergic reaction or other serious injury after receiving the COVID-19 vaccine, you cannot bring a claim against Pfizer, Moderna, or Johnson & Johnson. Under the Public Readiness and Emergency Preparedness Act (PREP Act), the federal government provides immunity from liability to vaccine manufacturers. There is an exception for willful misconduct but it is unlikely to apply to administration of the COVID-19 vaccine.
The PREP Act established a program that provides benefits to eligible individuals who suffer serious injury resulting from actions taken by a company that maintains protection from immunity under the Act. The Countermeasures Injury Compensation Program (CICP) provides up to $50,000 per year in reimbursed lost wages and out-of-pocket medical expenses. That said, the program is difficult to navigate and has rejected a majority of claims filed since the program began 10 years ago. It remains to be seen whether the COVID-19 vaccines will be added to the list of vaccines eligible for compensation under the National Vaccine Injury Compensation Program.
Lastly, COVID-19 vaccine attorneys are unable to pursue a claim against the FDA for authorizing the vaccine for emergency use, or against employers that mandate inoculation as a condition of employment.
I still have questions. Can I reach out to a COVID-19 vaccine injury lawyer?
Please note that, while we anticipate a change soon, injuries related to the COVID-19 vaccine are not yet covered under the National Vaccine Injury Compensation Program (VICP). We recommend checking out the website for the Counter Measures Injury Compensation Program at hrsa.gov/cicp.
If you have experienced a bad reaction to a vaccine other than the COVID-19 vaccine, contact The Law Offices of Leah V. Durant & Associates with your questions or concerns. We have been representing clients in VICP claims for more than eight years.
